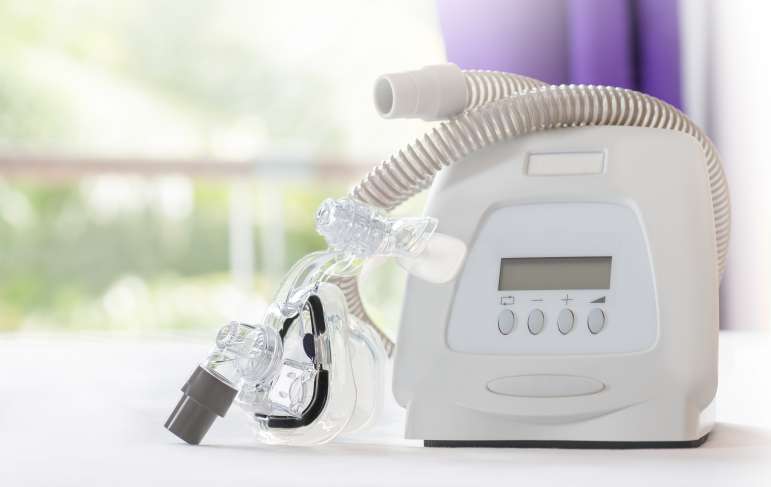
Senator Calls for Action Against Philips Respironics
U.S. Senator Richard Blumenthal recently expanded his call to action against Philips Respironics. He sent a letter to Federal regulators demanding aggressive enforcement against the company due to it not warning the public about a dangerous defect in its breathing machines. He sent a letter to U.S. Food and Drug Commissioner Robert M. Catliff and Attorney General Merrick Garland citing multiple articles about the company selling millions of sleep apnea machines when it knew that foam inside the machines was breaking down and emitting dangerous chemicals. The Senator called for the authorities to investigate these allegations thoroughly and to take action, including criminal charges if warranted.
Mr. Blumenthal last week called for the Justice Department to take action. He is a member of the Senate Judiciary committee and is chairman of a subcommittee that probes violations of laws and regulations that impact national health and safety. The letter calls for them to deter future wrongdoing by holding the company accountable. It was found that Philips kept over 3,700 complaints about the devices being faulty over an 11-year period secret. It just launched a recall two years ago of 15 million machines.
When the company initially recalled the devices, it claimed the foam releases chemicals or breaks down into small particles that are then inhaled, leading to life-threatening injuries. Now the company has reversed previous claims, saying that chemical emissions fall within safety standards. The FDA challenged these claims, saying that the tests were not adequate, and that Philips already agreed to perform other tests. The foam that degrades has been used since 2009.
Philips recently agreed to pay a settlement of $479 million due to the toxic foam. The money is going to people who bought or rented the devices and those who paid out of pocket costs or had to reimburse those for out-of-pocket costs. Around 10.8 million devices were recalled and there have been reports of at least 385 deaths associated with the broken-down foam. Philips Respironics claims that it has conducted risk assessments of 95 percent of devices covered by the recall and has concluded that exposure to foam particles and emissions is unlikely to result in appreciable harm to health.
Lawyers for the company claim the settlement amount will go up once more devices are returned. Awards will be paid to users for each recalled device that is returned, which will range from $55.63 to $1,552.25. A device return award of $100 will be given for every device given back to the company to cover the cost of a new machine. Six hundred and fifteen million dollars, or 575 million Euros, was set aside to cover these estimated costs. These claims are unrelated to claims of personal injury and medical monitoring costs.
Were you exposed to toxic foam or another toxic substance? Contact us today top see if you could be entitled to compensation. Fill out our contact form or call 412-471-3980 and a member of our team will get back to you as soon as possible.