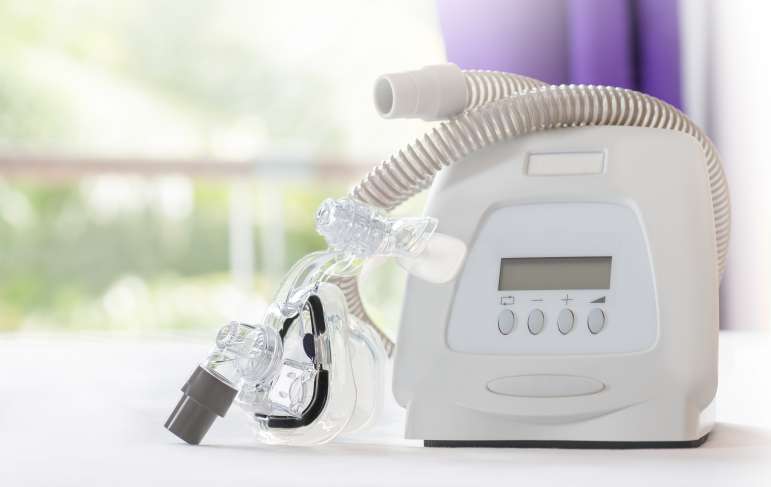
Philips CPAP and BiPAP Machines Have Been Recalled
Certain Philips Respironics, BiPAP, and CPAP machines have been recalled. The sound dampening foam that is made from polyester based polyurethane can break down and enter the devices’ air pathway. Either black debris or chemicals released in the air can be inhaled or swallowed by the user. Seek advice from your doctor if you use one of the affected devices.
Affected Devices
CPAP and BiPAP Devices
- Continuous Ventilator, Minimum Ventilatory Support, Facility Use
- E30 (Emergency Use Authorization)
- Continuous Ventilator, Non-life Supporting
- DreamStation ASV
- DreamStation ST, AVAPS
- SystemOne ASV4
- C-Series ASV
- C-Series S/T and AVAPS
- OmniLab Advanced+
- Noncontinuous Ventilator
- SystemOne (Q-Series)
- DreamStation
- DreamStation Go
- Dorma 400
- Dorma 500
- REMstar SE Auto
Ventilators
- Continuous Ventilator
- Trilogy 100
- Trilogy 200
- Garbin Plus, Aeris, LifeVent
- Continuous Ventilator, Minimum Ventilatory Support, Facility Use
- A-Series BiPAP Hybrid A30 (not marketed in US)
- A-Series BiPAP V30 Auto
- Continuous Ventilator, Non-life Supporting
- A-Series BiPAP A40
- A-Series BiPAP A30
If you use one of these devices, speak with a health care provider to find the best option for you. You can either stop using the device, use a similar device not part of the recall, continue using the device if the provider determines it is the best option for you, use alternative treatments like positional therapy or oral devices, or start long term mitigation procedures like losing weight, avoiding alcohol, stopping smoking, and considering surgery.
The manufacturers’ instructions for cleaning also need to be followed because certain cleaning techniques can make the foam degrade faster. This can occur with ozone and ultraviolet light cleaners and accessories. You should also register the device with Philips Respironics to be updated about your specific device.
BiPAP and CPAP machines are used to treat sleep apnea, which is a breathing disorder that affects three to seven percent of the population. While sleeping, people with sleep apnea stop breathing repeatedly because their throat muscles relax, the brain doesn’t send proper signals for breathing while sleeping, or a combination of the two. BiPAP stands for bilevel positive airway pressure. These machines pump air pressure to the airway. CPAP stands for continuous airway pressure machine that works by pumping air into the airway, keeping it open while people sleep.
The polyester based polyurethane foam in these devices is used to reduce sound and vibration and can be found in other medical equipment. The foam can potentially break down into small particles, entering the air pathway leading to people potentially swallowing or inhaling them into their lungs. Certain chemicals can also be released into the airway. Both cases can lead to serious injury, death, and permanent impairment.
The breakdown of the foam can lead to headache, upper airway irritation, cough, and chest pressure. It can also lead to irritation of the skin, eye, respiratory tract, inflammatory response, headache, asthma, and carcinogenic effect to organs including the kidneys and liver. Chemical exposure from the breakdown of the foam can lead to headache, dizziness, eye, nose, skin, and respiratory tract irritation, nausea and vomiting, and carcinogenic effects. If the foam is exposed to high humidity or unapproved cleaning methods like ozone, the degradation can happen much faster.
If you use one of these devices, speak to your healthcare provider to see what you should do about it. Don’t stop using or change your device without first speaking to your doctor. You can also seek advice about using an inline bacterial filter, which could help filter out particles in addition to bacteria. The filter will not help with chemicals and there is no guarantee that the filter will filter out particulate though. It could also affect performance because the air flow is restricted when a filter is used. When using these devices, look for foam debris on the filter or resistance problems when breathing.
If you use one of the affected devices and are experiencing problems, you could be entitled to compensation. Contact us today by calling 412-471-3980 or fill out our contact form and one of our employees will get back to you as soon as possible.